Cystine and cysteine are both amino acids containing sulfur. Both amino acids have different structures and functions in biological systems. Here are the key differences between Cystine and cysteine.
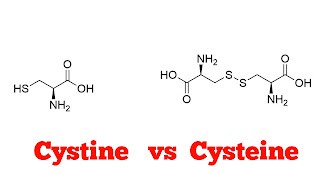 |
| Cystine and Cysteine Structure |
|
(1) Chemical Structure.
Cystine. Cystine is a dimer of cysteine. Meaning it consists of two cysteine molecules linked together by a disulfide bond (-S-S-).
Cysteine. Cysteine is a monomeric amino acid with a functional group of thiol (-SH).
(2) Formation.
Cystine. It forms when two cysteine molecules come close together and undergo oxidation. Their oxidation leads to the formation of a covalent bond between their sulfur atoms.
Cysteine. It can be synthesized within the body or obtained from dietary sources. Cysteine is the precursor to cystine, and it's involved in various biochemical processes.
(3) Function.
Cystine. Cystine is a structural component in proteins, particularly in proteins with a high sulfur content. Examples of proteins with high sulfur content include keratin which is found in hair, skin, and nails.
Cysteine. Cysteine plays diverse roles in biological systems. It is crucial for the formation of disulfide bonds in proteins which help stabilize their structure. Additionally, cysteine is involved in antioxidant defense mechanisms. Because cysteine is a precursor to the tripeptide glutathione, a potent antioxidant.
(4) Solubility.
Cystine. Cystine is less soluble in water compared to cysteine due to its dimeric structure.
Cysteine. Cysteine is more soluble in water because it exists as a single molecule.
(5) Stability.
Cystine. Due to its disulfide bond, cystine is more stable than cysteine under oxidizing conditions.
Cysteine. Cysteine is relatively unstable and can undergo oxidation to form cystine when exposed to air or other oxidizing agents.
FAQ About Cystine vs Cysteine.
(1) Difference Between Cysteine and Methionine.
Cysteine and methionine are two sulfur-containing amino acids with distinct roles in the body. Cysteine contains a thiol group (-SH). The Thiol group is important for forming strong bonds in proteins and for making antioxidants like glutathione. Methionine, on the other hand, is the initiator amino acid for protein synthesis. It plays an important role in metabolism by donating its methyl group. Unlike cysteine, methionine lacks a thiol group but contains a sulfur atom.
Cysteine contributes to protein structure and antioxidant defenses. On the other hand, methionine is primarily involved in protein production and metabolism. In summary, cysteine is important for protein structure and antioxidants. On the other hand, methionine is essential for starting protein synthesis and metabolic processes.
(2) Is Cysteine An Essential Amino Acid?
Cysteine is a semi-essential or conditionally essential amino acid. This means that under normal conditions, the body can synthesize cysteine from other amino acids. The amino acids from which our body can make cysteine include methionine and serine.
However, in certain conditions such as illness, stress, or inadequate dietary intake of cysteine precursors. Cysteine may become essential. In such conditions, it must be obtained from the diet. Cysteine is important for protein synthesis in the body. It is also crucial in antioxidant defense and various metabolic processes in the body.
(3) Cystine vs Cystine Stones.
Cystine stones are a type of kidney stone. They are caused by a genetic disorder called cystinuria. In cystinuria, there is a defect in the transport of cystine and other amino acids across the kidney tubules. This leads to high concentrations of cystine in the urine. When the concentration of cystine exceeds its solubility limit, it can precipitate and form crystals. Small crystals may eventually grow into stones.
Treatment for cystine stones often involves increasing fluid intake to dilute the urine. It also involves medications to alkalinize the urine and make cystine more soluble. In some cases, surgery may be necessary to remove large stones.
In summary, cysteine and cystine are amino acids with important roles in the body. However excessive cystine can lead to the formation of cystine stones in the urinary tract.
(4) Is Cystine More Stable Than Cysteine?
Yes, cystine is more stable than cysteine. Think of cysteine as a single Lego block, and cystine as two Lego blocks stuck together. Cysteine can sometimes be a bit like a loose block. On the other hand, cysteine being two blocks bonded together, tends to hold its shape better.
Cysteine can easily react with other molecules or itself. So, it can form bonds and change its structure. But when two cysteine molecules join together to form cystine, they create a stronger bond. This makes cystine less likely to break apart or react with other molecules.
So, in simple terms, cystine is a stronger, more stable form of cysteine. Because cystine is made of two cysteine molecules bonded together.
(5 How is cystine converted to cysteine?
Cystine is converted to cysteine through a process called reduction. This process involves breaking the disulfide bond that holds the two cysteine molecules together in cystine. Enzymes called reductases facilitate this reaction by providing the energy needed to break the bond. Once the bond is broken, cystine is converted into two cysteine molecules. These molecules can then be used by the body for various purposes.
In summary, cystine and cysteine differ in their structures, formations, functions, solubility, and stability. Cystine is a dimeric amino acid formed from two cysteine molecules linked by a disulfide bond. On the other hand, cysteine is a monomeric amino acid with a thiol group. Both play important roles in biological systems. In biological systems, cysteine serves as a versatile amino acid involved in various biochemical processes. On the other hand, cystine primarily contributes to the structural integrity of proteins.








0 Comments
Kindly share your views